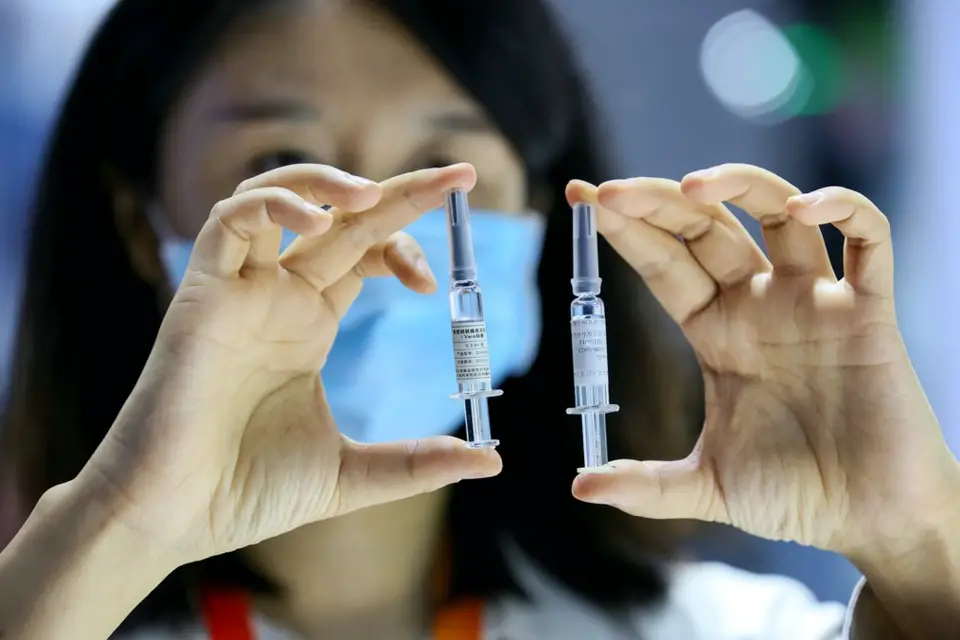
Staff members shows the coronavirus vaccine by the China National Biotec Group at the The China International Fair for Trade in Services (CIFTIS) held in Beijing on September 7. Photo by Luo Wei/People’s Daily Online
By Global Times
China's drug regulator has given conditional approval for the mass use of two additional COVID-19 vaccines produced by Chinese pharmaceutical firms, bringing the total number of home-produced vaccines granted conditional approval for mass use to four.
Vaccines developed by Sinopharm's research institute in Wuhan, Central China's Hubei Province, and another produced by CanSino have been granted conditional approval for mass use, according to a statement posted on the website of China's National Medical Products Administration on Thursday.
The approvals were granted under an emergency protocol and the two companies will continue to conduct research, meet the conditions required by the administration and submit research results in a timely manner.
Tao Lina, a Shanghai-based expert on vaccines and a former employee at the Shanghai Disease Prevention and Control, told the Global Times that the authorization on Thursday makes China so far the world's only country to have COVID-19 vaccines approved for mass use from two technological routes, adding a new option to the efforts to control the pandemic.
Tao noted that more options mean more flexibility in the assessment work, something other countries don't enjoy at the moment.
Pakistan officials mentioned in early February that CanSino's COVID-19 recombinant adenovirus vector vaccine showed 90.98 percent efficacy rate in preventing severe disease in interim analysis and is effective to prevent 65.7 percent of symptomatic diseases in clinical trials conducted in Pakistan and other countries.
Sinopharm's research institute in Wuhan said this week that their inactivated COVID-19 vaccine shows an efficacy of 72.51 percent after two shots in phase III clinical trials.
Previously, two homegrown vaccines have already been granted approval by the health authorities.
An inactivated vaccine developed by Beijing Biological Products Institute under Sinopharm's subsidiary, China National Biotec Group (CNBG), was China's first COVID-19 vaccine to receive conditional approval for the country's domestic market on December 31, 2020. It was followed by CoronaVac, developed by Chinese pharmaceutical firm Sinovac Biotech.
Source: Global Times
Vaccines developed by Sinopharm's research institute in Wuhan, Central China's Hubei Province, and another produced by CanSino have been granted conditional approval for mass use, according to a statement posted on the website of China's National Medical Products Administration on Thursday.
The approvals were granted under an emergency protocol and the two companies will continue to conduct research, meet the conditions required by the administration and submit research results in a timely manner.
Tao Lina, a Shanghai-based expert on vaccines and a former employee at the Shanghai Disease Prevention and Control, told the Global Times that the authorization on Thursday makes China so far the world's only country to have COVID-19 vaccines approved for mass use from two technological routes, adding a new option to the efforts to control the pandemic.
Tao noted that more options mean more flexibility in the assessment work, something other countries don't enjoy at the moment.
Pakistan officials mentioned in early February that CanSino's COVID-19 recombinant adenovirus vector vaccine showed 90.98 percent efficacy rate in preventing severe disease in interim analysis and is effective to prevent 65.7 percent of symptomatic diseases in clinical trials conducted in Pakistan and other countries.
Sinopharm's research institute in Wuhan said this week that their inactivated COVID-19 vaccine shows an efficacy of 72.51 percent after two shots in phase III clinical trials.
Previously, two homegrown vaccines have already been granted approval by the health authorities.
An inactivated vaccine developed by Beijing Biological Products Institute under Sinopharm's subsidiary, China National Biotec Group (CNBG), was China's first COVID-19 vaccine to receive conditional approval for the country's domestic market on December 31, 2020. It was followed by CoronaVac, developed by Chinese pharmaceutical firm Sinovac Biotech.
Source: Global Times
 Menu
Menu
 Two more COVID-19 vaccines get conditional approval for mass use in China
Two more COVID-19 vaccines get conditional approval for mass use in China